Remember the Huawei Watch D? It’s the brand’s first ever smartwatch to come with an ECG feature and built-in blood pressure monitor that was launched in China last year. And as we mentioned in our hands-on article back in May, Huawei has been aiming to release the device in Malaysia sometime in 2022, but only upon approval of local authorities. For those who’ve been anticipating its arrival, we have some good news.
After a check on the Malaysian Medical Device Authority Register (MDAR) database, we’ve discovered that both the smartwatch’s ECG and blood pressure monitor have been recently listed. More specifically, the two instruments received their approvals from the authority just last month on 15 November 2022.
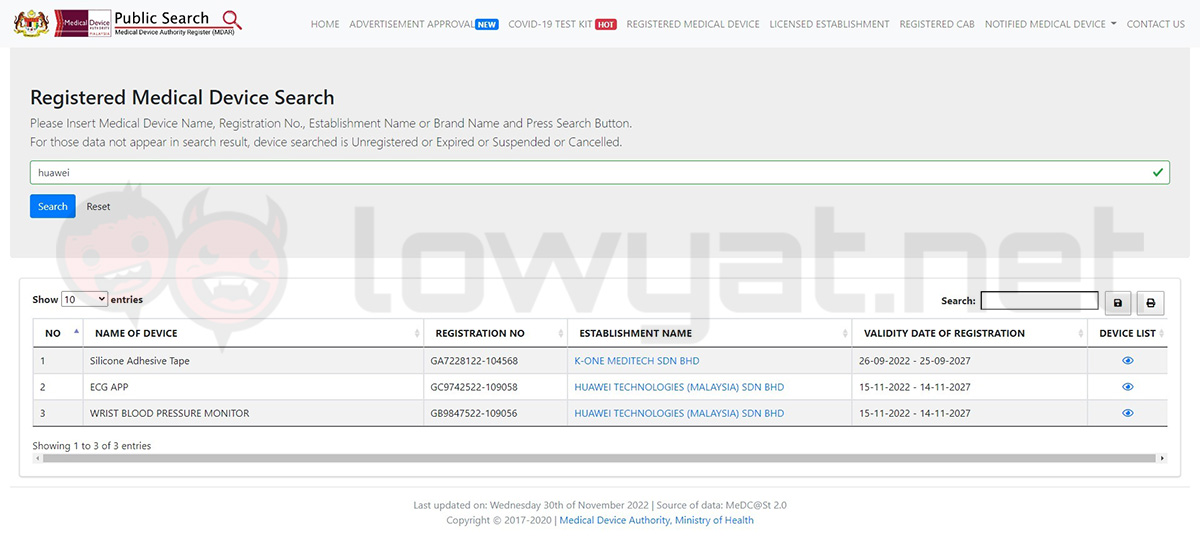
For the uninitiated, both the ECG and blood pressure monitor are considered as a tool to monitor medical conditions, therefore require approval from authorities before they are allowed to be distributed in a country as part of a product – in this case, a smartwatch. As you may recall, it also took a while before anyone in Malaysia could finally access the former feature on the Apple Watch Series 4 and above after their respective launches.
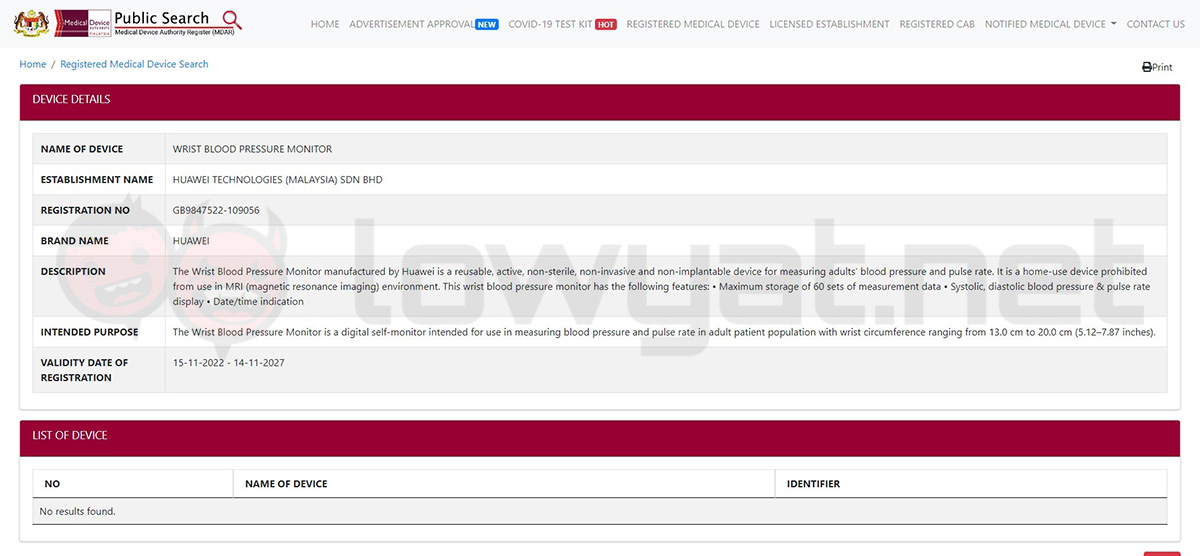
In Huawei’s case, the company decided to wait for a complete approval for the Watch D and its features instead of launching the device for the local market first. Which makes sense, as the main draw of the smartwatch is its built-in blood pressure monitor – a feature that has not been seen in any other consumer-based wearable just yet. That being said, despite being a year-old product, the Watch D’s arrival to regions outside of China could still bring a significant change to the smartwatch segment.
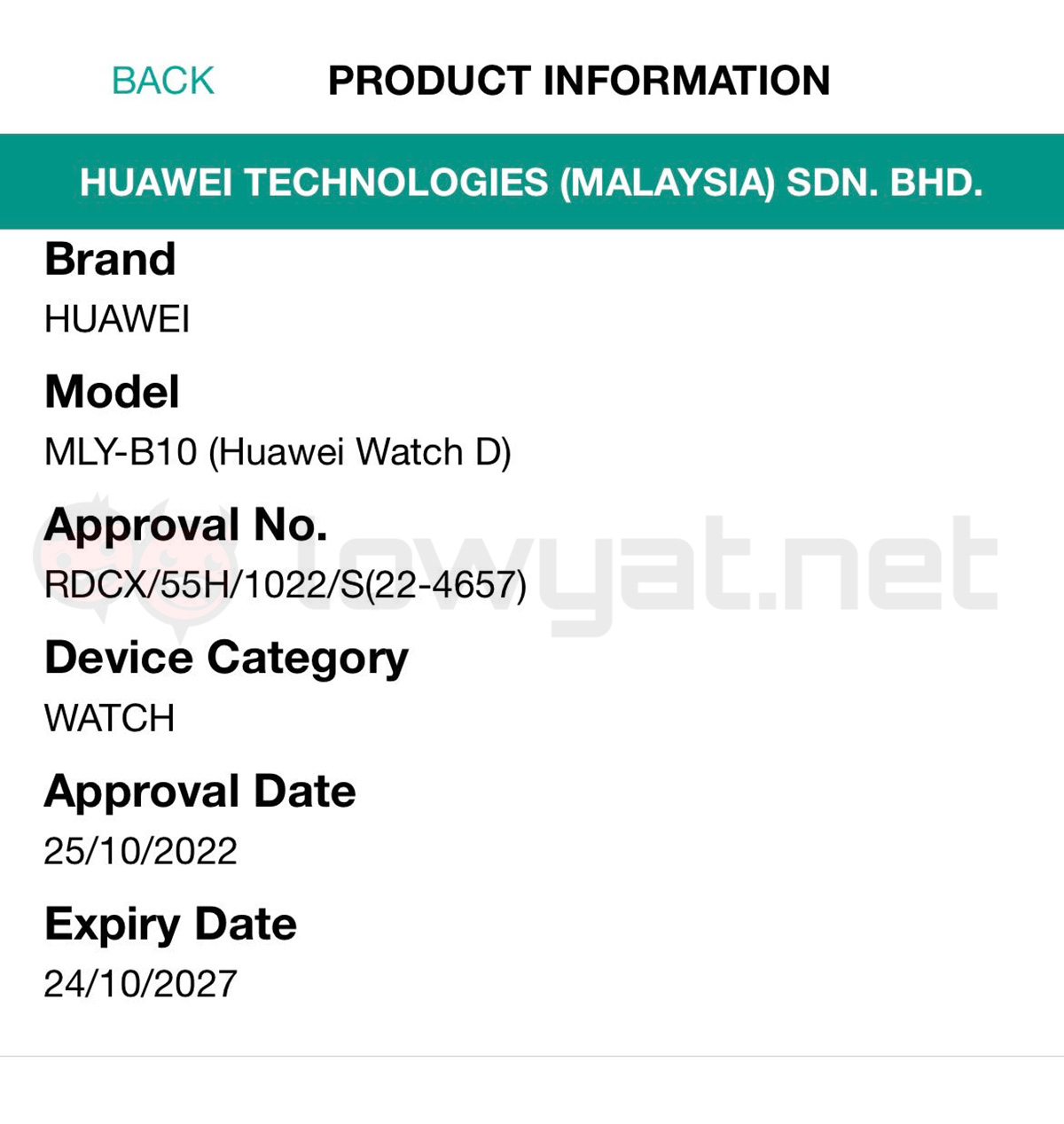
Additionally, we’ve discovered that the device has been approved by SIRIM as well, further corroborating on its imminent arrival in Malaysia. Based on our findings via MCMC’s Check Your Label mobile app, the Watch D’s listing was added in the organisation’s database back in late October this year.

As to when the blood pressure monitor-equipped wearable is expected to launch in the country, Huawei is still being quiet regarding that detail. Nevertheless, an official announcement could be made in the coming days or weeks, so stay tuned for that.
(Source: Medical Device Authority Register [1] [2] / SIRIM, via Check Your Label App)
Follow us on Instagram, Facebook, Twitter or Telegram for more updates and breaking news.



