Chinese pharmaceutical giant Sinovac Biotech has chosen Malaysia as one of the sites to conduct phase III trials of their COVID-19 vaccines on children under 12. Deputy Health Minister II Aaron Ago Dagang announced in Parliament today that the Sinovac trials on Malaysian children would begin by the end of October.
The clinical trials will be conducted at ten research locations involving eight facilities under the Health Ministry, Universiti Malaya, and UiTM with the involvement of the Institute for Clinical Research (ICR). He also mentioned that the US-developed Novavax vaccine, which has yet to be given conditional approval in Malaysia, was also involved in trials but gave no further details.
Aaron said that China had already approved COVID-19 vaccines from Sinovac and Sinopharm for emergency use on children between three and 17 years old. The minister added that phase I and II trials had shown that the vaccines were safe and increased immunity reaction among children and adolescents.
The Health Ministry has apparently also been in contact with several vaccine manufacturers to get their latest data on vaccines for those below 12. While Malaysia still does not allow administering the vaccine for those in that age group, the ministry is already in talks with manufacturers to procure vaccines for children.
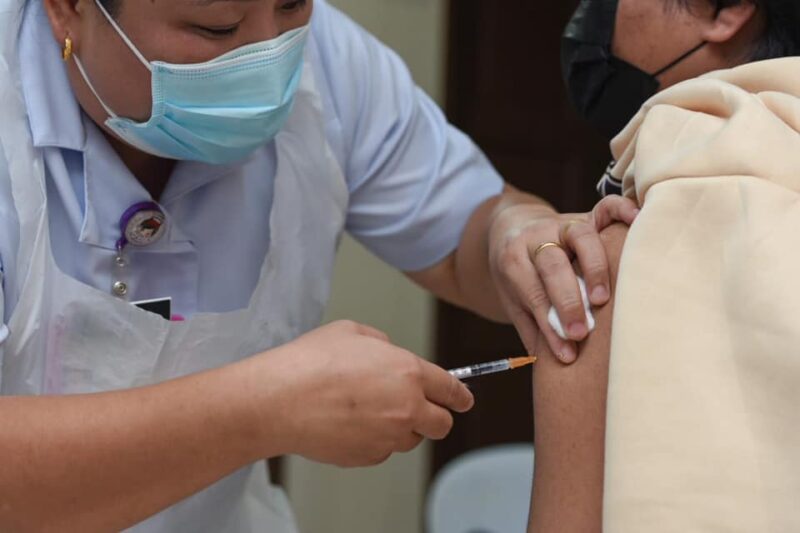
Pfizer had recently published its studies on their COVID-19 vaccine efficacy on children between the ages of five and 11 and found that it was 90.7% effective even with a much smaller dose. Former health minister Dr Dzulkefly Ahmad urged the government to take note of the US Food and Drug Administration (FDA)’s upcoming decision on whether to allow Pfizer-BioNTech shots to be used on kids, which is expected to come out later tonight at 8.30 PM.
(Source: The Star)
Follow us on Instagram, Facebook, Twitter or Telegram for more updates and breaking news.