The Beright Covid-19 Antigen Rapid Test Device has recently received conditional approval from the Ministry of Health (MOH) through the Medical Devices Authority (MDA), therefore making it the third self-test kit available for the public. This follows the earlier announcement of two other test kits, namely Salixium and Gmate, that were the first to be given the conditional approval.
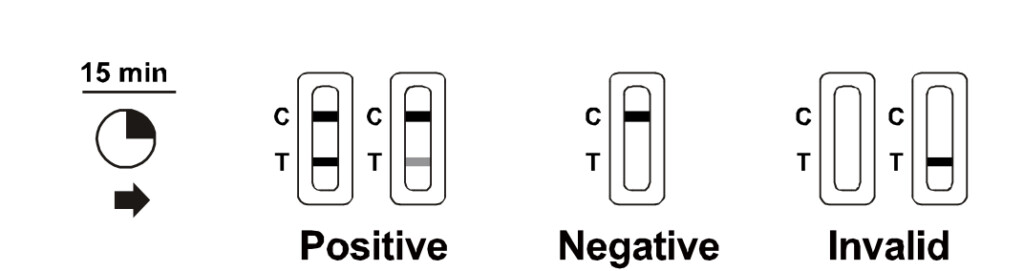
The Beright is manufactured by China’s Hangzhou AllTest Biotech and will be distributed here in Malaysia by Medinics. Just like Gmate, this RTK test kit only uses a saliva sample from the user and would take 15 minutes to produce results. The instructions say that the results should not be interpreted after 20 minutes, and users are required to dispose the kit in the provided biohazard bag after use.
According to Medinics, Beright has a sensitivity rate of 90.1% and a specificity rate of 99.3%. Salixium and Gmate, on the other hand, have a sensitivity rating of 91.23% and 91.0% respectively, with both having a specificity rating of 100%. A perfect specificity rating means that it’s unlikely to get a false negative, while the sensitivity rating shows that there’s a slight possibility of getting a false positive. The latter is reason why the MDA advices those who receive a positive result to follow up with an RT-PCR test for a second opinion.
Medinics’ website lists the newly approved oral self-test kit as “coming soon”, and there has been no word on pricing and availability at this time. Using the other two self-test kits as reference, it is possible that its price will also sport a RM39.90 price tag. Interestingly, the website also lists a nasal-swab version from Beright which is still pending for approval by the MDA.
As a gentle reminder, those who received a positive result should report themselves through MySejahtera’s Helpdesk. Salixium distributor, MeDKAD, announced that their test kit will have integration with MySejahtera through a companion app and that each kit comes with a unique QR code. However, the company has yet to launch the aforementioned app.
(Sources: The Star, MDA, Medinics [1][2], Reszon)
Follow us on Instagram, Facebook, Twitter or Telegram for more updates and breaking news.



