Following last week’s announcement of phase 5 of the vaccination drive, the National Pharmaceutical Regulatory Agency (NPRA) has approved the use of the Pfizer-BioNTech Covid-19 vaccine on children aged 12 and above.
“Although these additional indications have been approved, the Ministry of Health is of the view that vaccination priority should still be for high-risk groups, in line with the existing policy set under the National Covid-19 Immunisation Program (PICK),” Dr. Noor Hisham, health director-general, mentioned in a press statement today.
So far, Pfizer has been the only vaccine manufacturer to submit a request to the NPRA for its vaccine to be used on children.
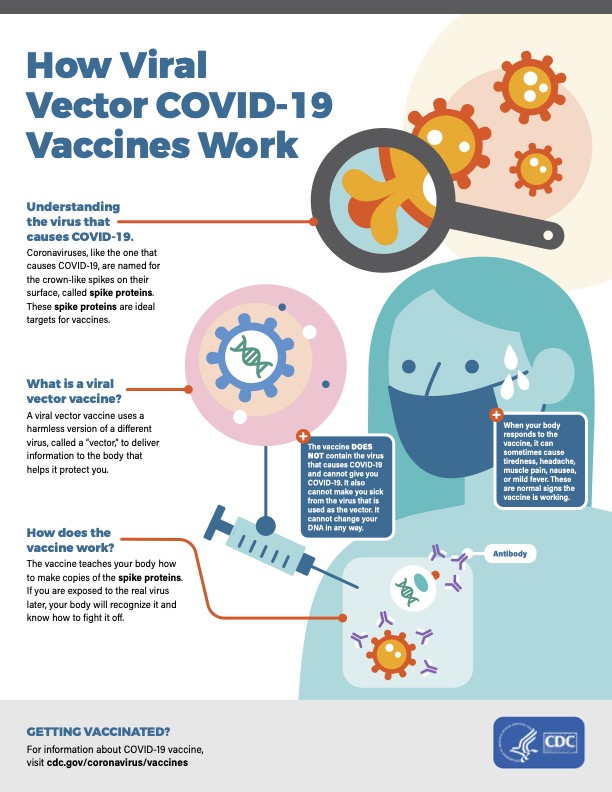
Dr. Noor Hisham also announced that the government has given conditional approval for the emergency use of the vaccines by CanSinoBio and Johnson & Johnson’s (J&J) Janssen, both of which only require a single dose to be fully vaccinated.
Both vaccines are viral vector types that use the adenovirus method and have similar efficacy rates. What’s noteworthy is that, unlike the Pfizer vaccine, neither the Sinovac nor J&J vaccine needs to be stored at ultra-low temperatures.
In March, Dr. Noor Azmi Ghazali, Deputy Health Minister, stated that the government may use single-dose vaccines in rural areas to account for the logistics and the rural communities.
“Those residing in interior areas may find it difficult to come again (to get a second vaccine shot),” he said.
Ghows Azzam, science advisor to Khairy Jamaluddin, mentioned in a tweet today that Solutions Biologics Sdn Bhd has registered the CanSino vaccine and will do the fill and finish in Malaysia. As for the J&J vaccine, its order details have yet to be revealed.
(Sources: CodeBlue, The Star, Bloomberg, CDC)
Follow us on Instagram, Facebook, Twitter or Telegram for more updates and breaking news.